Research
B cells are white blood cells that produce antibodies that protect our bodies against pathogens like viruses and bacteria. When a B cell spots a pathogen, it is triggered to multiply so that it can produce enough antibodies to neutralise the threat. This process of B cell activation relies on communication with antigen-presenting cells (APCs), such as follicular dendritic cells and subcapsular sinus macrophages. Research in our lab focuses on two interrelated questions: (1) How do mechanical forces in the immune synapse regulate B cell receptor (BCR) function, and (2) How do physical properties of APC membranes alter forces transmitted to BCR-antigen bonds. To answer these questions, we use a combination of quantitative imaging to visualise protein dynamics with high spatial and temporal resolution, DNA-based tension sensors to quantify molecular forces, and molecular and cell biology approaches to genetically perturb the system.
B cell receptor (BCR) mechanosensing
B cells recognise pathogenic antigens using their B cell receptors (BCRs). Because antigen recognition occurs in B cell-APC contacts, cellular forces are transmitted to BCR-antigen bonds. Mechanical forces alter bond lifetimes, BCR signalling, and BCR discrimination of antigen affinities. We use DNA-based molecular tension sensors to quantify the impact of mechanical forces on BCR function in the immune synapse.
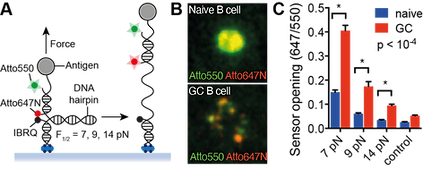
A) DNA-based tension sensor. B) Molecular maps of BCR-antigen forces during activation of a naive B cell and a germinal centre (GC) B cell. C) Quantitation of naive and GC B cell synaptic forces. (Nat Immunol 2016)
Physical properties of APC membranes
The forces transmitted to BCR-antigen bonds are influenced by physical properties of APC membranes. To build more realistic artificial surfaces for biophysical studies, we need to know the stiffness, mobility, and spatial organisation of the APC membranes that present antigens to B cells. We use quantitative imaging to assess these properties of primary follicular dendritic cells and subcapsular sinus macrophages, two APC types that present antigens to B cells in lymph nodes.

Live-cell images showing that the actin cytoskeleton of subcapsular sinus macrophages controls the mobility and clustering of immune complexes (multivalent antigens) presented on the cell surface. (bioRxiv 2022)
B cell antigen internalisation
B cells must extract and internalise antigens from APC membranes to fully activate. They do this using mechanical force. We develop DNA-based tools to study B cell antigen internalisation, processing, and trafficking. We are interested in how physical properties of the immune synapse influence these processes.

The quality and quantity of antigen that B cells internalise in the immune synapse depends on the stiffness of the antigen-presenting membrane. PLB: planar lipid bilayer (stiff membrane); PMS: plasma membrane sheet (flexible membrane). (J Cell Biol 2017)